Quality
We are dedicated to delivering excellence in all our endeavors. We consistently uphold superior standards in every facet of our engagement with partners and customers, meticulously attending to the complete collaborative experience.
Our unwavering dedication to quality, compliance, and innovation has earned us a reputation as a trusted partner in the healthcare industry. To achieve this, we have established a comprehensive quality management system that encompasses every stage of production, from sourcing raw materials to the final packaging. We work exclusively with pre-qualified manufacturers possessing state-of-the-art laboratories and testing facilities. The facility shall be equipped with the latest technology to ensure accurate and consistent results. Continuous monitoring and rigorous quality control protocols guarantee that only products of the highest standards bear our name.

Accreditations

ISO 9001:2015

ISO 13485:2016
We are audited for compliance on annual basis
Our Quality Management System complies with ISO 13485:2016 and ISO 9001:2015.
To comply with International Standards for Pharmaceuticals, We work exclusively with pre-qualified manufacturers and all our products comply with an official monograph in the most recent edition of the British Pharmacopoeia (BP), the US Pharmacopoeia (USP), and Ph.EUR where applicable. Also we follow ICH guidelines and respective country guideline for pharmaceutical product registration.
Regulatory Affairs:
Securing product registration across our export markets stands as a paramount objective, underscored by our extensive product registrations spanning more than 15 countries globally. Our proactive approach involves an ongoing evaluation and enhancement of our standards and capabilities to proactively anticipate regulatory shifts. Our unwavering dedication is reflected in both the meticulous upkeep of existing registrations and the continual addition of new registrations to our ever-growing roster. We have 200 products registered in 15 markets. And around 50 products in the pipeline.
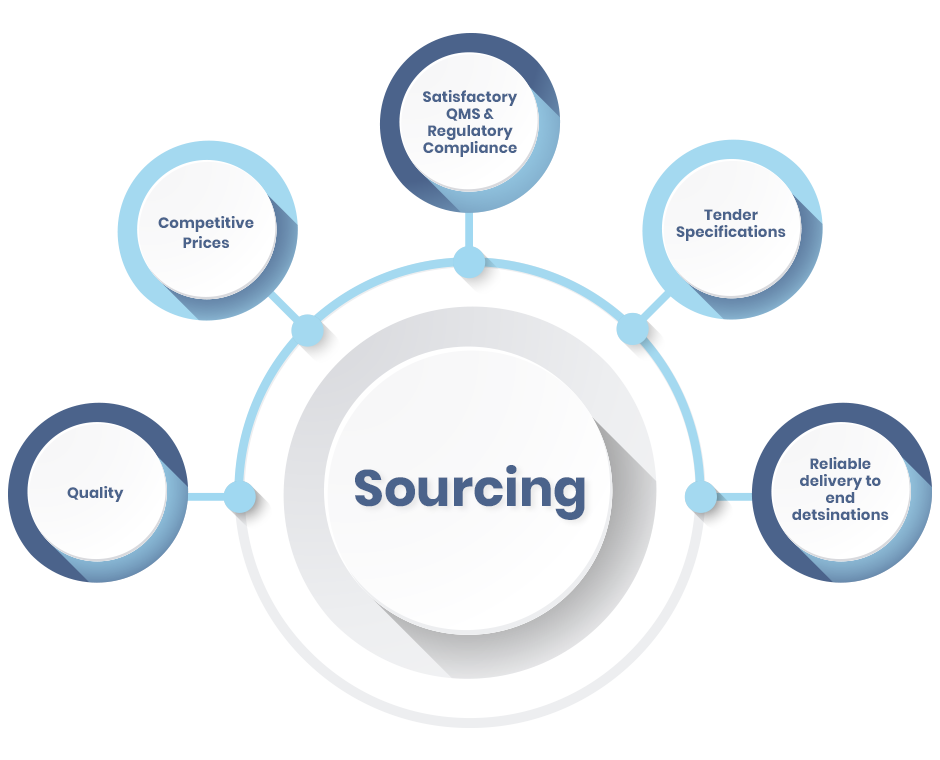
Optimizing Quality through Comprehensive Activities
Our activities include sourcing, inspection of manufacturers, GMP audits, on-site product QC, regulatory affairs, pre-shipment inspections and a range of procurement, logistics and support activities. These activities enable us to optimize our quality standards continually.
1. Lorem Ipsum
Avian remains a Single-point-of-contact approach throughout the entire project. Avian conducts procurement of thousands of products from India, Malaysia and China. Close contact with partners and huge sale volume assess us to also justify the need of small orders.
We have a dedicated team of procurement experts based in India. We build strong and enduring partnerships with manufacturers, sharing knowledge and experience for mutual gain. We are committed to consistently delivering the best products at fair prices.
India is a world pharmacy; therefore we get the opportunity and access to work with various manufacturers as per the country specific requirements.
RIGHT MARKET - RIGHT PRODUCT - RIGHT PRICE - RIGHT ACCREDITATION
- Our selected suppliers are a natural fit for the market, which is why we choose to work with them
- We prioritize quality over cost, and this is why we maintain long-term relationships with our suppliers.
- Our suppliers hold various essential accreditations, such as EU GMP, WHO GMP, FMACA, ANVISA, NDA (Uganda), PPB, Philippine FDA, Zambia (ZAMSA), and TFDA (Tanzania), ensuring their credibility and compliance.
- We enter into market-exclusive agreements to secure an ample market share.
- Brand exclusivity agreements are also established to protect our brands.
- Avian plays a critical role in market access, pharmacovigilance, and product recalls
- Clear roles and responsibilities are defined for both the manufacturer and Avian to ensure efficient product supply.
- Territory-specific conditions are outlined in our agreements
- Payment terms are pre-established with our suppliers, providing financial clarity
- Our agreements often include provisions to mitigate losses in case of handling issues, ensuring our financial security
- Avian is a reliable source because all parties involved are obligated to adhere to the terms and conditions set in the agreements
- Avian is committed to visiting supplier facilities during the manufacturing process to verify timely production and product quality.
- We maintain a wide network of suppliers to provide flexibility and options in sourcing products, enhancing our ability to meet market demands effectively
Logistics
Ensuring the safe delivery of our products to medical facilities and pharmacies is a complex task that AVIAN makes effortless. We have the expertise to manage both small-scale projects and oversee all aspects of large-volume, complex endeavors.

Our logistic offerings are defined by a capacity to
- We guarantee timely deliveries within specified timeframes
- We discern the optimal port for swift and budget-friendly shipments
- Regard logistic remedies as an inherent component of our value offers
- Furnish customized answers to best match customer requisites
- Sustain intimate collaboration with the client during the whole logistical continuum
- We carefully choose the shipment route
AVIAN enhances cargo supervision by collaborating with a select group of global freight forwarders. This approach allows us to achieve heightened cost efficiency through the consolidation of volumes, all the while harnessing their specialized knowledge and regional connections.
AVIAN enhances cargo supervision by collaborating with a select group of global freight forwarders. This approach allows us to achieve heightened cost efficiency through the consolidation of volumes, all the while harnessing their specialized knowledge and regional connections.
Local Partnerships
We have a team of local agents handling the warehousing at Uzbekistan.



We are also expanding our reach to creating warehousing facilities and creating a newer segment for Avian where Avian can handle the final distribution to end users.